Researchers at UCLA have developed a new way to separate and organize cells suspended in fluid samples by their subtle biochemical differences. The system sorts cells more quickly and accurately than current methods, and could lead to a simple, rapid automation of cell analysis, as well as an easier way to separate therapeutic cells from non-therapeutic, or “contaminating,” cells.
Cell sorting is widely used in life sciences research and in diagnostic and industrial processing. For example, it is used to isolate progenitor or stem cells from tissues or in vitro cultures; these cells can then be delivered back to a patient to heal injuries or attack tumor cells. The magnetic ratcheting system developed at UCLA can distinguish between subtly different cells so that only the correct, therapeutic cells are used for treatments.
“What we think is only one cell type is often a heterogeneous mixture, and without technologies to separate quantitatively, these nuanced differences get lost,” said Dino Di Carlo, the principal investigator on the research and a professor of bioengineering at the UCLA Henry Samueli School of Engineering and Applied Science. “For example, therapeutically active progenitor cells may look very similar to the other contaminating cells that provide no therapeutic benefit.”
Currently, there are two common cell-sorting techniques. One technique is to use fluorescence to find target cells; this requires a large number of cells to complete an analysis because many are damaged or lost during the process. It’s also relatively slow. The other uses magnetic tagging and isolation; this method is faster, but typically can provide only a binary “yes” or “no” analysis, identifying only two types of cells. This process may let through many non-targeted cells, contaminating the end result.
The new system, described in a new study published in the journal Small, combines the speed and gentle processing of magnetic sorting with the precision and range of sorting by fluorescent-based techniques.
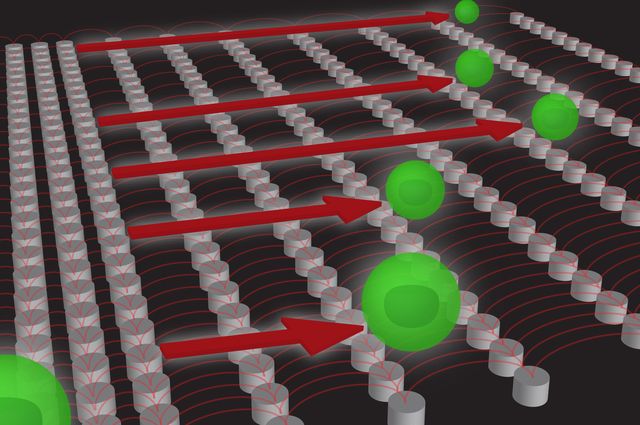
The system comprises an array of microscale pillars that are progressively farther apart, and a spinning magnet that pulls magnetically tagged cells over the pillars. The more magnetic tags a cell has, the farther it is pulled along the array. The cells then settle into defined groups based on its magnetic tags, which correspond to biochemical markers on the surface of the cell. Researchers can then identify the cells and see how many of each type there are, as well as performing further analyses.
“Imagine hurdles on a running track that get progressively higher,” said Coleman Murray, the study’s lead author and a postdoctoral scholar in bioengineering at UCLA. “Each set of hurdles holds back those hurdlers who cannot clear the height. The cells are sorted in a similar manner — those with more magnetic tags will be forced past more of the micropillar hurdles.”
The researchers see this magnetic ratcheting technology as a new approach to an automated system that could fit on a glass chip, known as a “lab on a chip,” where samples such as blood could be rapidly analyzed with resolutions as fine as single cells.
The study’s co-authors are Edward Pao, Peter Tseng, Shayan Aftab, all current or former members of Di Carlo’s Biomicrofluidics Lab at UCLA Bioengineering; and Dr. Rajan Kulkani and Dr. Matthew Rettig of the UCLA David Geffen School of Medicine and the UCLA Jonsson Comprehensive Cancer Center.
The research was supported through the National Institutes of Health and the UCLA Clinical Translational Sciences Institute. Di Carlo is also a member of the California NanoSystems Institute and the Jonsson Comprehensive Cancer Center.