Key takeaways
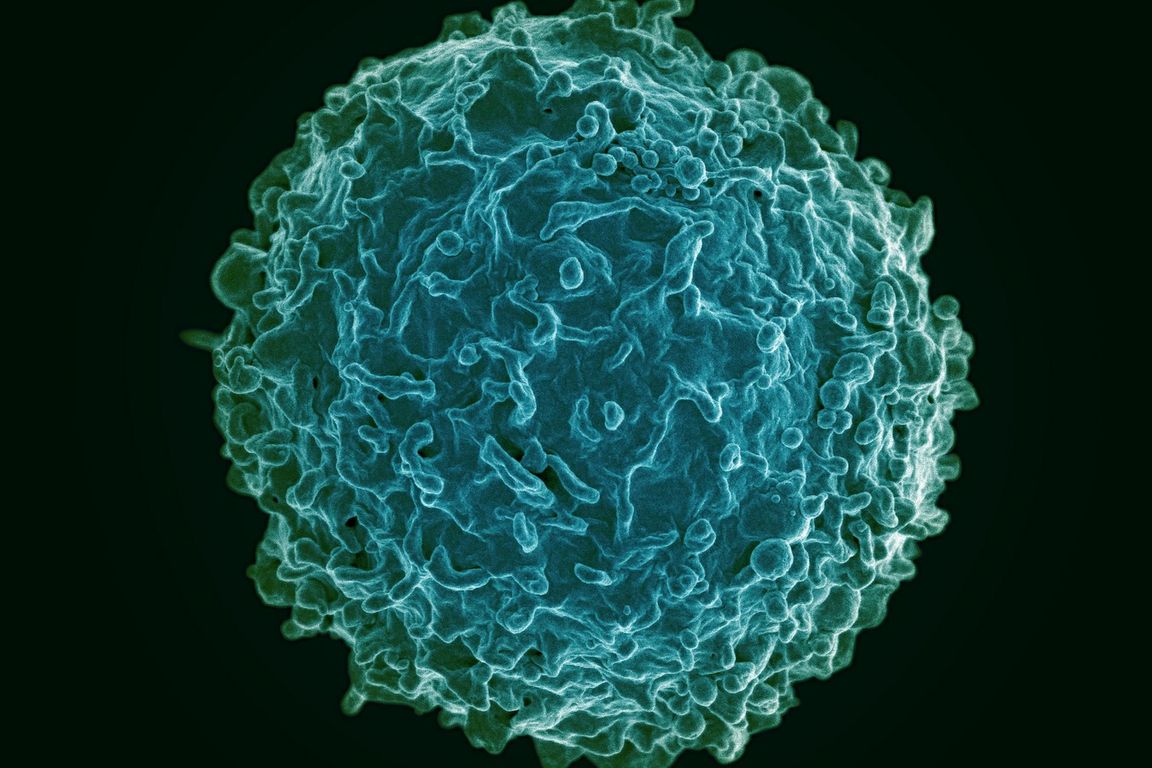
- B cells, white blood cells that play a crucial role in fighting infections in the body, use mechanical force to pull antigen molecules off of other cells.
- This process helps B cells assess which immune cells can best target each antigen with antibodies and then produce more of those cells.
- The use of mechanical force helps B cells learn, adapt and evolve quickly to confront new pathogens and remember them while continuing to combat known pathogens.
Most cells evolve slowly, accumulating incremental changes that better suit their environments. Immune cells, because they must adapt rapidly to counter new threats, evolve much more quickly. Part of that, UCLA physicists now report, rests on their ability to forcibly pull antigens off other cells’ surfaces and “study” them.
By using this type of mechanical force, the immune system’s B cells, which create antibodies that fight off harmful pathogens like viruses, bacteria and parasites by targeting their antigens, are able to better gauge the properties of particular antigens, compare which types of cells among the B cell population recognize and respond to each antigen most efficiently, and produce more of those cells.
The discovery that immune cells use mechanical force — in addition to biochemical signaling — in an active, deliberate way to advance their own evolution adds a new paradigm to studies of immune system learning and memory, said Shenshen Wang, a UCLA assistant professor of physics and astronomy and corresponding author of the research.
“We showed that fast-evolving immune cells use tactile sense, by applying active tugging forces, to learn about their antigenic targets and to rank themselves,” Wang said. “Such active physical sensing allows the immune cell repertoire to respond effectively to current challenges while being plastic and adaptive against future threats.”
That cells sense and respond to external physical forces has long been a cornerstone of biology. But the knowledge that they generate their own physical forces to acquire signals is recent, with evolutionary consequences that haven’t been explored until now. Wang said the findings may help scientists figure out how to guide the evolution of the immune system by designing a sequence of vaccines to help it learn to identify and encode the most important features of different antigens. An immune system tuned this way could, for example, recognize and neutralize a virus it has never encountered before much more quickly.
The research is published in two peer-reviewed journal papers. In Proceedings of the National Academy of Sciences, Wang and her co-author, UCLA physics doctoral student Hongda Jiang, describe how B cells pull antigens from the antigen-presenting cells to which they are tethered, generating mechanical stress that propagates through connected cell surfaces and changes the distribution of energy at the points where cells touch each other.
Even when B cells’ attempts to extract an antigen from a particularly stiff antigen-presenting cell are not successful, Wang and Jiang note, the process still enhances B cell learning. More importantly, the deformation of the connected structure by mechanical force increases the amount of time immune cells can “remember” what they’ve learned and extends the range of antigens they can detect. And if pulling gets stronger over time, the detection range expands significantly.
In a paper accepted for publication in the open-access journal Physical Review X, Wang and Jiang address an immune cell dilemma: How the body’s finite set of immune B cells achieves a balance between responding with sufficient strength to antigens they’ve already encountered while also being able to recognize pathogens they’ve never seen before. The new research shows that B cells’ mechanical force–driven evolution improves memory diversity, giving natural immunity greater adaptive potential by balancing the potency of response to antigens with the breadth of coverage.
By opening a wide variety of biophysical pathways to improve antigen-recognition ability, this active process gives the body’s natural immunity more ways to adapt to new mutants and future variants. The authors write that the process works hand-in-hand with biomechanical sensing and biochemical signaling to produce nimble immune systems able to rapidly evolve to cope with new challenges.
“We are proposing a new paradigm of biological recognition via physical acquisition of stimuli, which may complement the current view centered on biochemical signaling,” Wang said. “Meanwhile, it offers a fresh angle for understanding biological adaptation in light of physical influences on evolution. Our findings have broad implications for understanding biological learning and for physically steering adaptive evolution in general, and immune response in particular.”